Answer: The type of bond that joins the carbon atom to each of the hydrogen atoms is called a covalent bond. A covalent bond is a type of chemical bond where two atoms share one or more pairs of electrons to achieve a stable configuration.
To understand how a covalent bond works, let’s look at an example of a simple molecule: methane (CH4).
Methane has one carbon atom and four hydrogen atoms. The carbon atom has four valence electrons, which are the outermost electrons that can participate in bonding. The hydrogen atoms have one valence electron each.
To form a covalent bond, the carbon atom needs to share its valence electrons with at least one other atom. It can do this by either:
- Sharing one pair of electrons with each hydrogen atom, forming single bonds (C-H). This way, each carbon-hydrogen pair has two shared electrons and two unshared electrons. The remaining unshared electrons on the carbon atom form a triple bond with another carbon atom in methane.
- Sharing two pairs of electrons with each hydrogen atom, forming double bonds (C=C). This way, each carbon-carbon pair has four shared electrons and no unshared electrons. The remaining unshared electrons on the carbon atoms form single bonds with other atoms in methane.
The figure below shows the molecular structure of methane and how it forms covalent bonds.
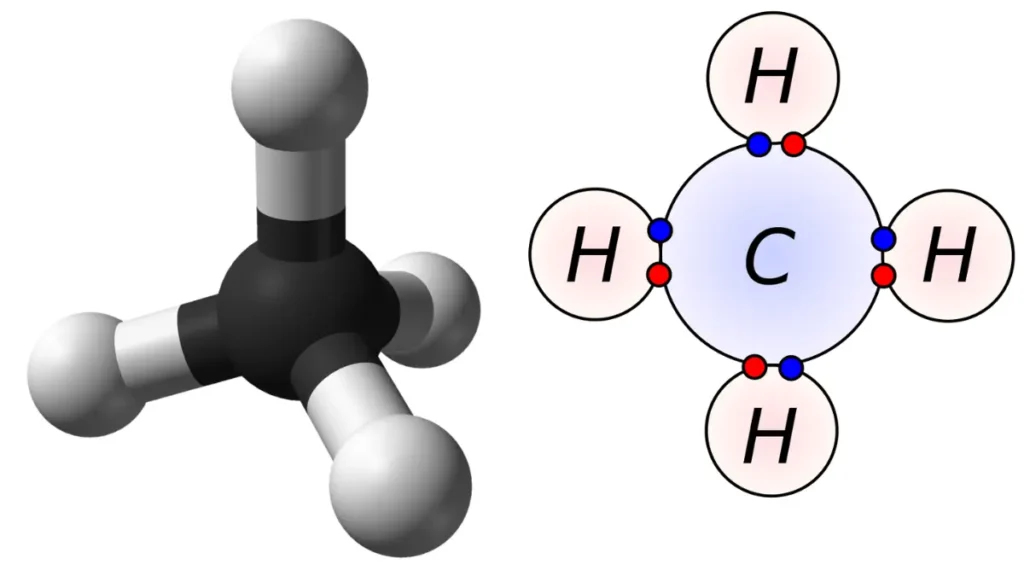
As you can see, the covalent bonds between the carbon and hydrogen atoms are represented 3D figure.